Submitted by aml95 on Thu, 24/10/2024 - 23:01
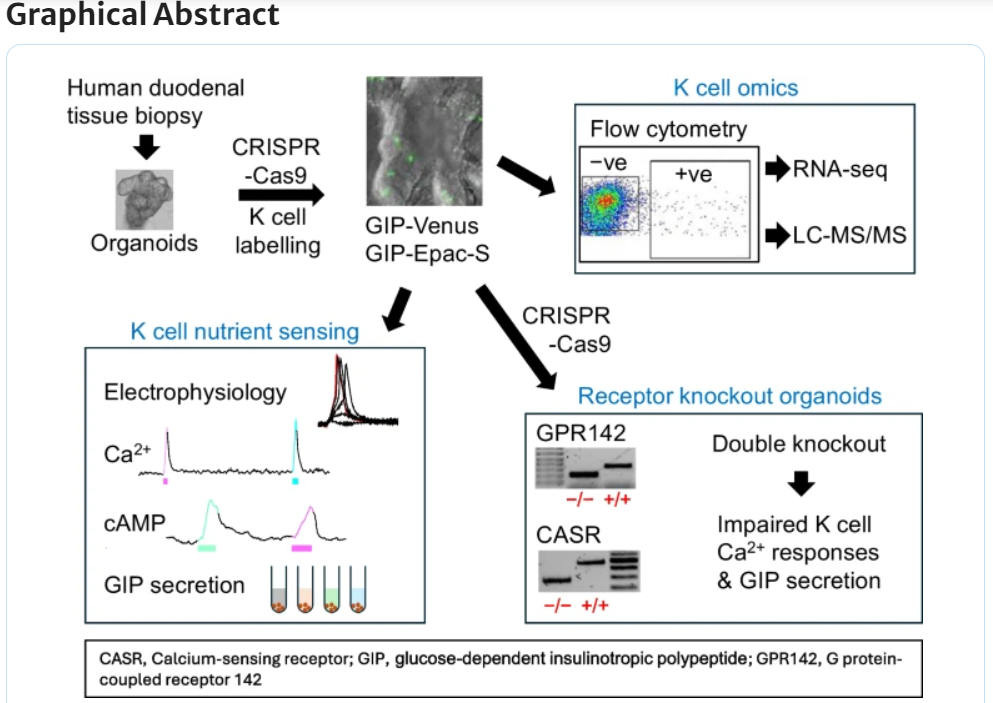
Research from the Gribble-Reimann group has generated a new human organoid K cell model which enables transcriptomic and functional characterisation of nutrient-sensing pathways involved in human GIP (glucose-dependent insulinotropic polypeptide - an incretin hormone secreted by enteroendocrine K cells in the proximal small intestine) secretion.
The study, published in Diabetologia, which aimed to explore the function of human K cells at the molecular and cellular levels, also showed both calcium-sensing receptor (CASR) and G protein-coupled receptor 142 (GPR142) contributed to protein-stimulated GIP secretion. This model will be further used to identify potential targets for modulation of native GIP secretion in diabetes and obesity.
Reference: Guccio, N., Alcaino, C., Miedzybrodzka, E.L. et al. Molecular mechanisms underlying glucose-dependent insulinotropic polypeptide secretion in human duodenal organoids. Diabetologia (2024). https://doi.org/10.1007/s00125-024-06293-3

